by Sandeep Nema (Editor), John D. Ludwig (Editor)
Parenteral Medications is a definitive reference utilized by academic institutions, government agencies, and the pharmaceutical industry. The first edition was published in 1984, and the second and third editions followed in 1991 and 2010, respectively.
Over the last decade, there has been a rapid, transformational change in the rate of innovation across pharmaceutical drug discovery and manufacturing sciences. History may well regard this period as a golden era of science. New technologies have allowed medicinal chemists to design highly optimized small-molecule therapeutics not possible at the turn of the century. Complex new biotherapeutic modalities have flourished bringing the promise of enhanced drug safety and efficacy to millions of patients.
These include monoclonal antibodies, bi-specific antibodies, tri-specific antibodies, antibody–drug conjugates, nanoparticles (and targeted nanoparticles), aptamers, RNAi therapeutics, viral vector delivery (gene medicine), chimeric antigen receptor T cells (CAR-T), DNA vaccines, and more. In addition, there have been significant advances in drug delivery, administration devices, analytical techniques (multi-attribute methods, cryoelectron microscopy), computational-based design (in silico modeling, machine learning), and manufacturing and control technologies (including continuous processing and real-time release).
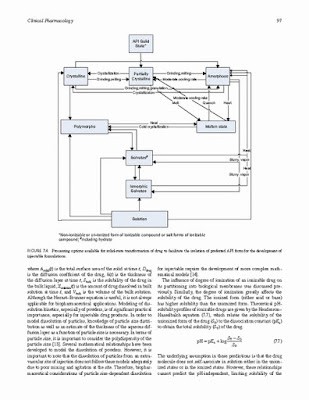
The global regulatory environment has shifted towards greater emphasis on science-based risk assessment as evidenced by the evolving Current Good Manufacturing Practices (cGMPs), quality by design (QbD), process analytical technology (PAT), and other initiatives. In addition, regulatory authorities have developed fast-track approval processes to advance critical new medicines to market with limited clinical data sets in order to address unmet medical need. This has resulted in greater pressure on the parenteral formulators and process engineers to lock down the manufacturing processes and product quality profiles early in the development cycle, often during Phase 2 clinical trials, to ensure rapid regulatory review and approval. There have also been unexpected headwinds in the parenterals marketplace, including a greater frequency of regulatory observations/warning letters at parenteral manufacturers, substantial injectable drug shortages, and sterility failures associated with “compounding pharmacies.”
This extraordinary changing landscape in the parenteral field was the primary reason we undertook the challenging task of updating the book. Our objectives for the fourth edition were to (1) document the emerging technologies and industry trends, (2) provide the most current sterile product development and manufacturing science, and (3) combine the three volumes from the previous edition into a single, integrated book more easily accessible by the reader.
The fourth edition not only reflects enhanced content in all the chapters, but more than a quarter of the chapters are new highlighting the rapidly advancing technology. We have divided the book into seven sections: Section 1: Parenteral Drug Administration and Delivery Devices Section 2: Formulation Design and Development Section 3: Specialized Drug Delivery Systems Section 4: Primary Packaging and Container Closure Integrity Section 5: Facility Design and Environmental Controls Section 6: Sterilization and Pharmaceutical Processing Section 7: Quality Testing and Regulatory Requirements
The authors invited to contribute chapters are established leaders with proven track records in their specialty areas. Hence, the textbook is authoritative and contains much of the collective experience gained in the (bio)pharmaceutical industry over the past 15 years. Practical guidance is provided, in addition to theoretical aspects, for how to bring a parenteral drug candidate forward from discovery, through preclinical and clinical development, manufacturing, scale-up, validation, and eventual registration. We are deeply grateful to all the authors who made this work possible.
We would like to express special gratitude to the late Kenneth E. Avis (University of Tennessee College of Pharmacy) for his dedication to teaching and sharing practical knowledge in the area of parenteral medications to so many students over the years, including us. Also, thanks to Rick J. Rutter (Pfizer, Inc. retired) whose commitment to investing in (1) new science and technology approaches and (2) an empowered workforce, both critical components to success in the parenteral field, enabled the editors to “deliver the projects” to waiting patients. Finally, thanks to Cathleen Hanau Taylor for being a phenomenal technical editor and Hilary Lafoe from Taylor & Francis Group for guiding us through the art and science of book publishing.
Product details
- Publisher : CRC Press; 4th edition (August 14, 2019)
- Language : English
- Hardcover : 1143 pages
- ISBN-10 : 1498719147
- ISBN-13 : 978-1498719148
- Item Weight : 6.98 pounds
- Dimensions : 8.5 x 2.3 x 11.1 inches






No comments:
Post a Comment